Research
Wheeler research group Word Cloud, based on publications and presentation titles through 2020
Research
Areas: Organic Supramolecular
Chemistry, Molecular Recognition,
Nanomaterials, Solid-State Organic
Photochemistry, and Crystal Engineering.
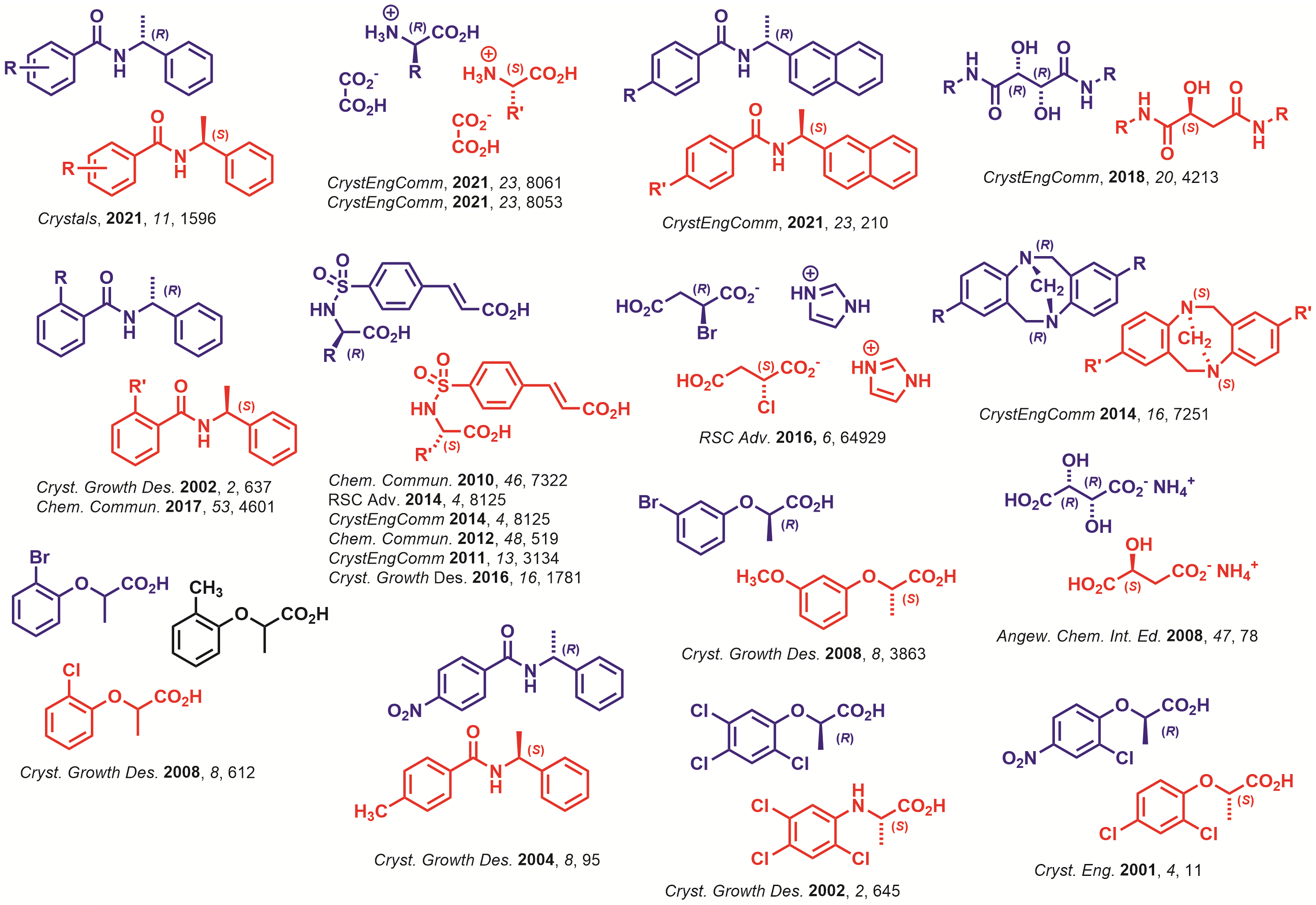
Structural Boundaries of Molecular Shape Based Molecular Recognition
compounds, conglomerates, and solid solutions) is possible given the unique thermal signature of each phase.
The collection of diarylamides selected for this initial investigation differed incrementally in size and shape that offered clear advantages for exploring the molecular recognition profiles that originate from topological features. Thermally processing all possible sets provided a comprehensive view of the shape space of this structural family. For example, the sets of H/F, Cl/Br, and Br/CF3 quasienantiomeric pairs showed the formation of quasiracemic compounds, while the H/CN and F/Cl systems showed only conglomerate formation. These results and others were recently highlighted in a CrystEngComm and ChemCommun articles.
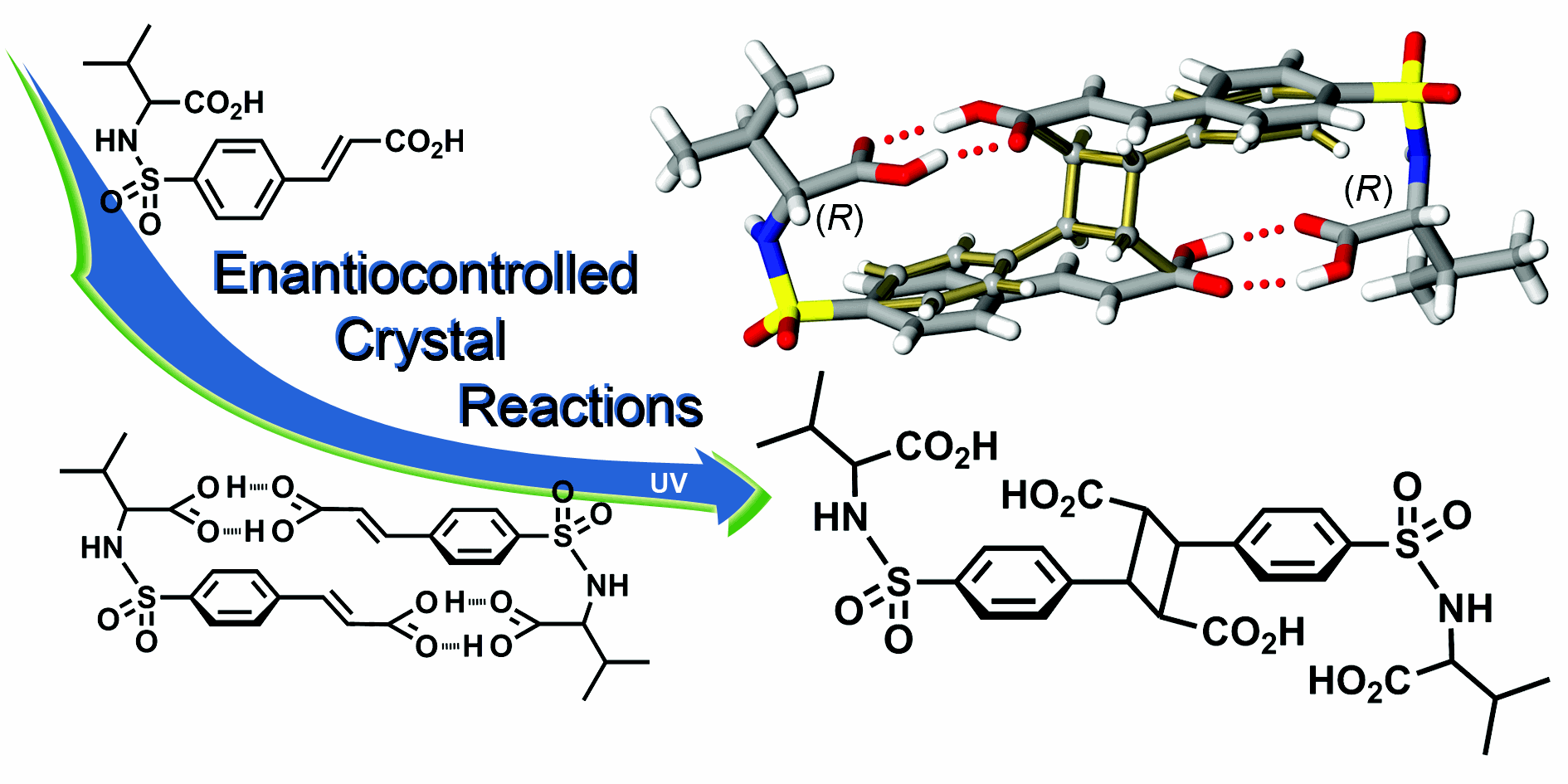
Asymmetric [2+2] Photodimerization Reactions in Molecular Crystals
The strategy of this program exploits sulfonamidecinnamic acid frameworks that form robust supramolecular dimers due to the features of molecular topology and directionality of hydrogen bonds. Several recent studies show these dimeric motifs persist regardless of the use of racemic, quasiracemic, and homochiral single-component building-blocks. Each of these systems undergoes topochemically controlled single-crystal-to-single-crystal (SCSC) photodimerization reactions in high yield (61–100% conversion). The current study presents several interesting opportunities to explore the supramolecular and photoreactive boundaries of this sulfonamidecinnamic acid framework.
This program extends the current crystal reaction technology to include the formation of robust molecular dimers that undergo asymmetric light-initiated reactions. Noted progress in this area has been realized by a team of researchers - undergraduates, a master’s student, and a high school teacher – all actively engaged in the design and development of a wide range of photoactive materials.
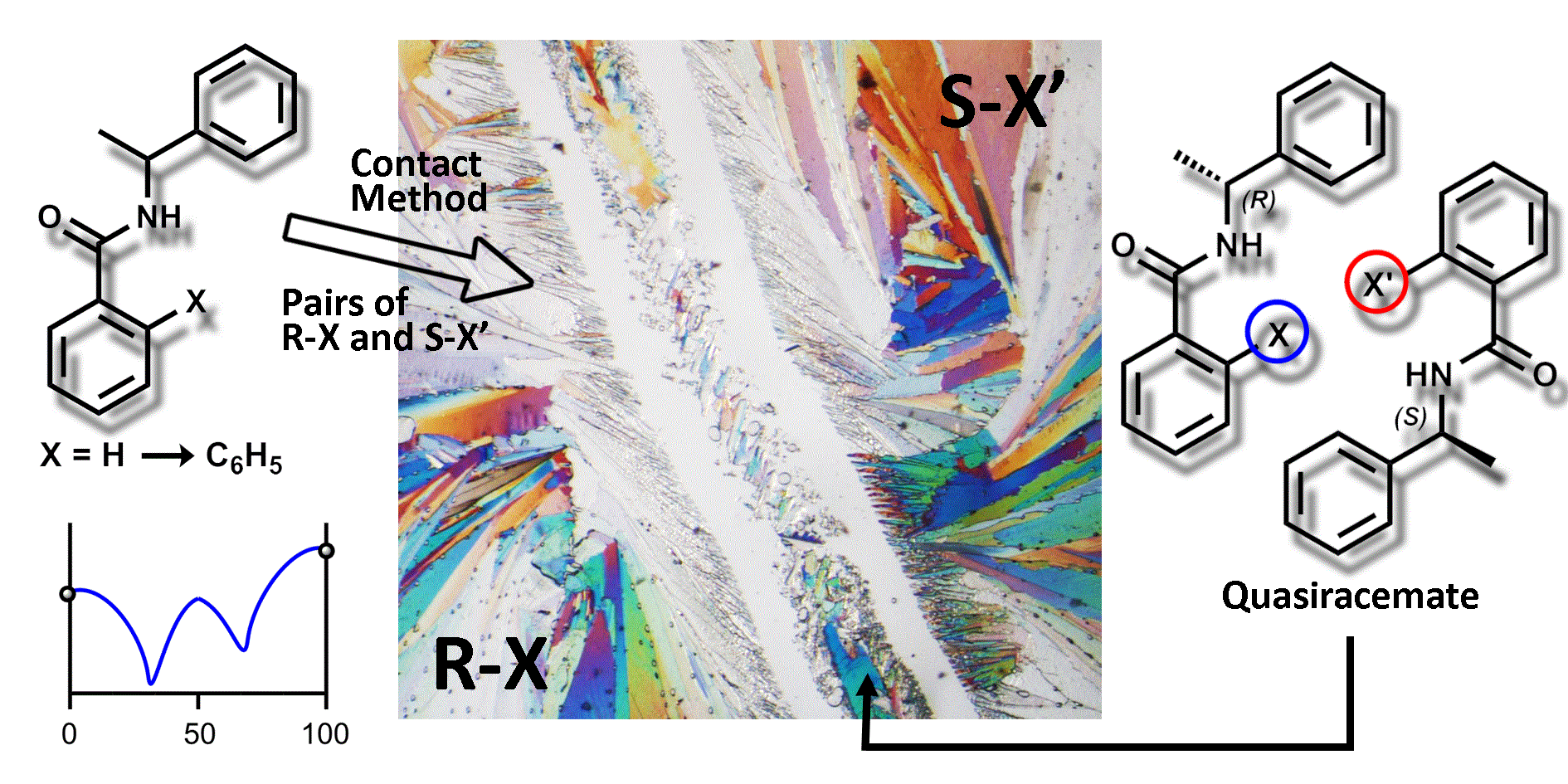
Quasiracemic Materials
Our recent interest in understanding the recognition profile of molecular topology makes use of the quasiracemate approach for organizing supramolecular arrays. It is generally accepted that quasiracemates are related to ‘true' racemates by an isosteric change in one of the components. When crystallized, they form supramolecular patterns with approximate inversion relationships that, in all known cases to date, mimic the structures of the corresponding racemate(s). One important advantage of this strategy is that it promotes the use of topological features rather than intermolecular electrostatic forces such as hydrogen bonds. In doing so, quasiracemic materials are not limited to specific chemical classes, and thus their construction may proceed using a wide variety of chemical frameworks and functions.
Our crystallographic investigations of quasiracemates have demonstrated that the deliberate use of isosteric components that differ in handedness consistently form supramolecular motifs that mimic the centrosymmetric alignment of racemic compounds. These systems include a variety of Some of our previously studies of quasiracemic systems are shown below and highlight the use of isosteric components
In summary, this collection of racemic and quasiracemic structures underscores the importance of molecular shape to the construction of supramolecular assemblies with near centrosymmetric arrangements. The deliberate use of phenoxypropionic acids that vary in Cl, Br, and CH3 functions provided a logical entry point to isosteric components that were assessed for quasiracemate behavior. The structures of both racemic and quasiracemic systems contain molecular dimers organized by carboxylic acid head-to-head interactions. In the case of each quasiracemate, construction of these discrete motifs occurs from pairs of isosteric components that closely mimic the centrosymmetric local environment observed for the racemates. Further assembly of these heterodimers generates two possible crystalline phases with space group P21 and Z' = 2 or 8. The structural themes of isostructurality (both local and crystal structure patterns) and concomitant polymorphism in these studies emphasize the inherent importance of molecular topology as a contributor to molecular recognition processes.